The Periodic Table Explained: Elements Made Simple
Explore the periodic table and its significance in chemistry. Learn about elements, their properties, periodic trends, and how the table organizes atoms to predict chemical behavior. Perfect for students and science enthusiasts.
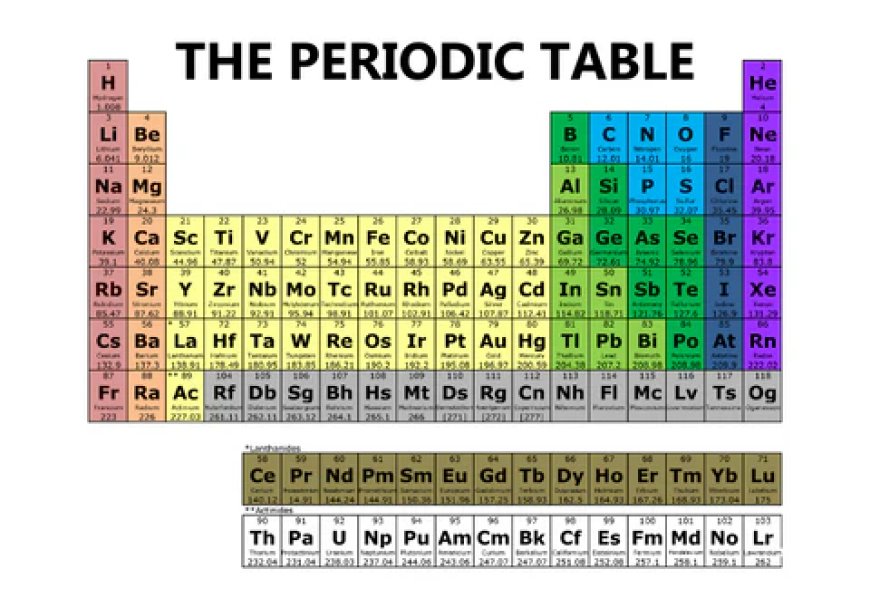
The periodic table is one of the most iconic symbols in science, representing the fundamental building blocks of everything around us. But what exactly makes it so important, and how does it work? This article will help simplify the periodic table and explain its significance in understanding the natural world.
Introduction to the Periodic Table
The periodic table is a systematic arrangement of chemical elements based on their atomic structure and properties. First introduced in 1869 by Russian chemist Dmitri Mendeleev, it has become an indispensable resource for scientists worldwide. Mendeleev’s genius lay in leaving gaps for yet-to-be-discovered elements, and his predictions were proven astonishingly accurate over time.
Why is the Periodic Table Important?
The periodic table serves as a fundamental tool in chemistry, helping scientists predict the behavior of elements and their interactions. It is not just a reference but a framework for understanding the relationships between elements, providing insights into everything from chemical reactions to atomic structure.
The Structure of the Periodic Table
At first glance, the periodic table might look like a simple grid, but it is far more than that. The structure is meticulously organized to reveal patterns and relationships among the elements. Understanding these patterns is key to understanding chemistry.
Periods and Groups
The periodic table is divided into two main components: periods and groups.
-
Periods: These are the horizontal rows of the table. Each period represents elements with the same number of electron shells. As you move from left to right across a period, the atomic number increases, reflecting the addition of protons and electrons.
-
Groups: These are the vertical columns. Elements in the same group have similar chemical properties because they share the same number of valence electrons (electrons in the outermost shell). This gives them similar reactivity and bonding characteristics.
Atomic Number and Its Role
The central organizing principle of the periodic table is the atomic number, which is the number of protons in an element's nucleus. As you move from left to right across a period, the atomic number increases by one for each successive element. This systematic arrangement allows scientists to predict the behavior of elements based on their position on the table.
Types of Elements
The periodic table categorizes elements into three main types: metals, nonmetals, and metalloids. Each category has its distinct properties that make it unique.
Metals
- Location: Metals are found predominantly on the left and center of the periodic table.
- Properties: Metals are shiny (luster), malleable (can be hammered into thin sheets), and excellent conductors of heat and electricity. Examples of metals include iron, gold, and aluminum.
- Uses: Metals are used in a wide range of applications, from construction materials to electrical wiring.
Nonmetals
- Location: Nonmetals are located on the right side of the table.
- Properties: Nonmetals lack the metallic qualities of luster and conductivity. They tend to be brittle in solid form and are poor conductors of heat and electricity. Examples include oxygen, nitrogen, and carbon.
- Uses: Nonmetals are essential for life processes, such as oxygen for respiration and carbon in organic compounds.
Metalloids
- Location: Metalloids are found along a zigzag line between metals and nonmetals.
- Properties: Metalloids exhibit properties of both metals and nonmetals. For example, silicon is a metalloid known for its semi-conductive properties, making it crucial in electronics and technology.
- Uses: Metalloids are valuable in the production of semiconductors, essential in the electronics industry.
Understanding Groups and Periods
The structure of groups and periods is the backbone of the periodic table, and understanding how they function is key to understanding the behavior of elements.
Groups: Similar Valence Electrons
- Group 1 (Alkali Metals): These elements all have one valence electron, making them highly reactive. Examples include lithium, sodium, and potassium.
- Group 2 (Alkaline Earth Metals): These elements have two valence electrons and are slightly less reactive than alkali metals. Examples include magnesium and calcium.
Periods: Changing Properties
As you move across a period, elements transition from metals to nonmetals. This shift in properties reflects the changes in atomic structure, such as the number of electron shells and the arrangement of electrons. Elements in the same period share the same number of electron shells but differ in their chemical properties.
The Periodic Trends
The periodic table is not just a chart; it’s a tool that helps scientists understand several key trends in element properties. These trends give us insights into the behavior of elements in different chemical reactions.
Atomic Size
- Across a Period: As you move from left to right, atomic size decreases. This happens because, as more protons are added to the nucleus, the positive charge pulls electrons closer to the nucleus, making the atom smaller.
- Down a Group: Atomic size increases as you move down a group because additional electron shells are added, making the atom larger.
Ionization Energy
Ionization energy refers to the energy required to remove an electron from an atom.
- Across a Period: Ionization energy increases from left to right due to the stronger attraction between the nucleus and electrons.
- Down a Group: Ionization energy decreases because outer electrons are farther from the nucleus, making them easier to remove.
Electronegativity
Electronegativity measures an atom’s ability to attract electrons in a chemical bond.
- Across a Period: Electronegativity increases as atoms gain more protons and attract electrons more strongly.
- Down a Group: Electronegativity decreases as atoms become larger and the attraction between the nucleus and electrons weakens.
Special Element Categories
Some groups of elements stand out due to their unique properties.
Noble Gases
- Location: Group 18
- Properties: Noble gases like helium, neon, and argon are chemically inert because they have complete electron shells. This makes them stable and unreactive under most conditions.
- Uses: Noble gases are used in lighting, such as neon signs, and in certain types of lasers.
Alkali Metals
- Location: Group 1
- Properties: Alkali metals are highly reactive, especially with water. They have one valence electron and are soft and shiny.
- Uses: Alkali metals are used in batteries and various chemical processes.
Transition Metals
- Location: The central block of the table
- Properties: Transition metals are known for their ability to form colorful compounds and serve as catalysts in industrial processes.
- Examples: Iron, copper, and gold are well-known transition metals.
The Importance of the Periodic Table
The periodic table is more than just a scientific reference; it’s a vital tool in multiple fields of study and industries. Its importance extends beyond the classroom into real-world applications.
1. Scientific Research
The periodic table is essential for understanding chemical behaviors, reactions, and the relationships between elements. Scientists use it to predict how elements will react in different conditions, which aids in advancing research across various scientific fields.
2. Education
The periodic table simplifies the complex world of chemistry, making it easier for students and educators to grasp fundamental concepts. Its systematic organization allows students to learn about trends, element properties, and how they relate to one another.
3. Industrial Applications
The periodic table is critical in industries such as materials science, electronics, and energy production. For example, semiconductors, which rely on the properties of elements like silicon, are fundamental in modern technology.
4. Medical Innovations
Elements like iodine and cobalt play significant roles in medical treatments, such as diagnostic imaging and cancer treatment.
5. Environmental Insights
Understanding the elements helps scientists tackle global challenges like pollution, resource management, and energy efficiency. The periodic table is a vital tool in addressing environmental concerns and finding sustainable solutions.
Conclusion
The periodic table is far more than just a chart—it’s a gateway to understanding the natural world. By organizing the elements, it helps us make sense of the materials that compose everything from the air we breathe to the stars in the sky. Whether you’re a student, a scientist, or simply curious, the periodic table invites you to explore the building blocks of matter and uncover the wonders they hold.
1. What is the simple way to understand the periodic table?
The periodic table is a chart that organizes all known elements based on their atomic number (number of protons). Elements are arranged in rows (called periods) and columns (called groups). The table helps us understand relationships between elements, including their properties, behaviors, and how they react with one another.
2. How do you explain the elements on the periodic table?
The elements on the periodic table are substances made up of atoms, each with a unique number of protons. Each element is represented by a symbol, which is usually one or two letters (e.g., O for oxygen, Fe for iron). The table is organized by atomic number, and elements in the same column (group) share similar chemical properties.
3. How to learn 1 to 30 elements easily?
To remember the first 30 elements:
- Mnemonics: Create a funny sentence where the first letter of each word corresponds to an element (e.g., "Happy Harry Hates Being Bored, Caught On Monday, Naughty Neighbors Ask."
- Flashcards: Write the element's name and symbol on one side and atomic number on the other side.
- Practice: Regularly quiz yourself or use apps that help with memorization.
4. What are elements explained simply?
Elements are pure substances that consist of only one type of atom. Each element is unique, and it cannot be broken down into a simpler substance. Examples include oxygen (O), hydrogen (H), and carbon (C).
5. How to teach the periodic table easily?
Start by explaining the periodic table as a map for elements. Emphasize that the rows (periods) indicate the number of electron shells, and the columns (groups) show how many electrons are in the outer shell (valence electrons). Use simple examples to show how groups and periods determine element properties.
6. What does O stand for on the periodic table?
O stands for oxygen, which is a chemical element with atomic number 8. Oxygen is essential for life on Earth, and it’s a part of water (H2O) and many other compounds.
7. How do you explain the periodic table to a child?
To explain the periodic table to a child, think of it as a big chart that groups similar things together. Just like how we have categories like animals, fruits, or vehicles, elements are grouped in similar ways based on their properties. For example, elements in one column are like "friends" that have similar traits.
8. How do you remember the periodic table easy method?
- Mnemonic Devices: Use catchy phrases or stories where the first letter of each word corresponds to the element symbols.
- Chunking: Break the table into smaller sections (e.g., the first 10 elements, then the next 10) and focus on memorizing in chunks.
9. What are the main points of a periodic table?
- Atomic Number: Each element has a unique atomic number that tells you the number of protons in its nucleus.
- Groups and Periods: Elements are arranged in periods (rows) and groups (columns), with each group sharing similar properties.
- Element Symbol: Each element has a one- or two-letter symbol.
- Atomic Mass: Most elements have a mass number listed, which is the combined number of protons and neutrons.
10. How do you tell how many electrons are in an element?
In a neutral atom, the number of electrons equals the atomic number. For example, if an element has an atomic number of 6 (like carbon), it has 6 electrons.
11. What are the basics of elements?
Elements are basic building blocks of matter. Each element consists of atoms, which are made up of protons, neutrons, and electrons. Each element has unique properties, such as atomic number, mass, and reactivity.
12. How to find protons, neutrons, and electrons?
- Protons: The number of protons equals the atomic number.
- Electrons: In a neutral atom, the number of electrons equals the number of protons.
- Neutrons: Neutrons can be found by subtracting the atomic number from the atomic mass (rounded to the nearest whole number).
13. What is the basic explanation of the periodic table?
The periodic table is a chart that organizes all known elements by their atomic number and properties. It shows how elements are related to one another and groups them based on shared characteristics, such as metals, nonmetals, and noble gases.
14. Will element 119 be discovered?
Element 119, also known as ununennium (Uue), is a predicted element, and scientists are working on creating it. It has not yet been discovered, but ongoing research in nuclear physics may lead to its discovery in the future.
15. How to define atomic number?
The atomic number is the number of protons in the nucleus of an atom. It is the defining feature of an element, and each element has a unique atomic number.
16. What is the easiest definition of the periodic table?
The periodic table is a chart that organizes elements based on their atomic number, and it groups elements with similar properties together.
17. How to read an element on the periodic table for beginners?
To read an element, look at its symbol, atomic number, and atomic mass. The symbol represents the element, the atomic number tells you how many protons it has, and the atomic mass tells you the total number of protons and neutrons.
18. What are 5 facts about the periodic table?
- It was created by Dmitri Mendeleev in 1869.
- Elements are arranged by increasing atomic number.
- It contains over 100 elements.
- Groups (columns) share similar chemical properties.
- The table is divided into metals, nonmetals, and metalloids.
19. What is the simple way of periodic table?
A simple way to think of the periodic table is as a chart that shows the "family" of elements. Elements are grouped by similar characteristics, with rows representing energy levels and columns representing elements that behave in similar ways.
20. What is RA in the periodic table?
RA refers to Radium, which is a radioactive element with the symbol Ra and atomic number 88. It is part of the alkaline earth metals.
21. How to find valence electrons?
To find the number of valence electrons, look at the group number of the element (if it is in groups 1-2 or 13-18). For example, oxygen (group 16) has 6 valence electrons, while sodium (group 1) has 1.
22. What is a simple definition of proton?
A proton is a positively charged particle found in the nucleus of an atom. The number of protons defines the element and determines its atomic number.
23. What are the names of the 18 groups in the periodic table?
- Alkali metals
- Alkaline earth metals
- Boron group
- Carbon group
- Nitrogen group
- Oxygen group
- Halogens
- Noble gases
- Lanthanides
- Actinides
- Transition metals (divided into multiple groups)
- Other main groups (grouped in the periodic table into groups 13-18)
24. Why is it called periodic?
The periodic table is called "periodic" because the elements exhibit recurring (periodic) properties when arranged by atomic number. These periodic trends repeat in a regular pattern across periods and groups.